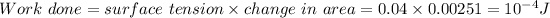Answer:
So amount of work produced will be

Step-by-step explanation:
We have given diameter of ammonia bubble is changes from 1 cm to 3 cm
So radius changes from 0.5 cm to 1.5 cm
Surface area of bubble
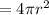
So change in area of bubble
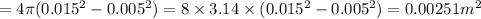
Surface tension of ammonia = 0.04 N/m
So work done will be