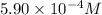The question is incomplete, here is the complete question:
A chemist makes 600. mL of magnesium fluoride working solution by adding distilled water to 230. mL of a stock solution of 0.00154 mol/L magnesium fluoride in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.
Answer: The concentration of chemist's working solution is
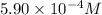
Step-by-step explanation:
To calculate the molarity of the diluted solution (chemist's working solution), we use the equation:

where,
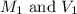 are the molarity and volume of the stock magnesium fluoride solution
are the molarity and volume of the stock magnesium fluoride solution
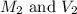 are the molarity and volume of chemist's magnesium fluoride solution
are the molarity and volume of chemist's magnesium fluoride solution
We are given:
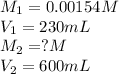
Putting values in above equation, we get:
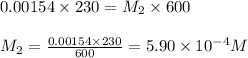
Hence, the concentration of chemist's working solution is