Answer: The percentage by mass of NaCl in the mixture is 38.5 %
Step-by-step explanation:
To calculate the mass percentage of element in compound, we use the equation:
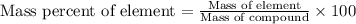 .......(1)
.......(1)
Mass of sodium element = 23 g
Mass of NaCl = 58.5 g
Putting values in equation 1, we get:
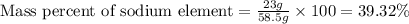
Mass fraction of sodium metal in NaCl = 0.3932
- For
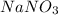 :
:
Mass of sodium element = 23 g
Mass of
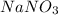 = 85 g
= 85 g
Putting values in equation 1, we get:
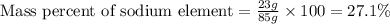
Mass fraction of sodium metal in sodium nitrate = 0.271
Let us assume the mass fraction of NaCl in the mixture is 'x'
So, the mass fraction of
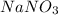 in the mixture will be '(1-x)'
in the mixture will be '(1-x)'
We are given:
Percent by mass of Na in the mixture = 31.8 %
Mass fraction of Na in the mixture = 0.318
Evaluating the mass fraction of NaCl in the mixture:
![[(x* 0.3932)+((1-x)* 0.271)]=0.318](https://img.qammunity.org/2021/formulas/chemistry/college/rh3jh59z6p0z6c7f1trgnx3oherylx2j7t.png)
x = 0.385
Percent by mass of NaCl in the mixture will be =
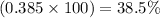
Hence, the percentage by mass of NaCl in the mixture is 38.5 %