The question is incomplete, here is the complete question:
Assuming that all the
 comes from HCl, how many grams of sodium hydrogen carbonate will totally neutralize the stomach acid? Volume = 500 mL pH= 2
comes from HCl, how many grams of sodium hydrogen carbonate will totally neutralize the stomach acid? Volume = 500 mL pH= 2
Answer: The mass of sodium hydrogen carbonate needed to completely neutralize stomach acid is 0.42 grams
Step-by-step explanation:
To calculate the hydrogen ion concentration of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
We are given:
pH = 2
Putting values in above equation, we get:
![2=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/28qonyl5c6mjl3bcxffx6ymuzd5ujtxvqu.png)
![[H^+]=10^(-2)M](https://img.qammunity.org/2021/formulas/chemistry/college/cdaisg89o1digjdrqare3cil3a5tnqwbv7.png)
- To calculate the number of moles for given molarity of solution, we use the equation:
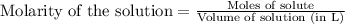
Molarity of hydrogen ions = 0.01 M
Volume of solution = 500 mL = 0.5 L (Conversion factor: 1 L = 1000 mL)
Putting values in above equation, we get:
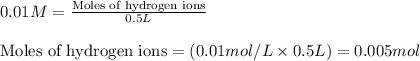
The chemical equation for the reaction of HCl and sodium hydrogen carbonate follows:

By Stoichiometry of the reaction:
1 mole of HCl reacts with 1 mole of sodium hydrogen carbonate
So, 0.005 moles of HCl will react with =
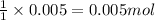 of sodium hydrogen carbonate
of sodium hydrogen carbonate
- To calculate the number of moles, we use the equation:

Moles of sodium hydrogen carbonate = 0.005 moles
Molar mass of sodium hydrogen carbonate = 84 g/mol
Putting values in above equation, we get:
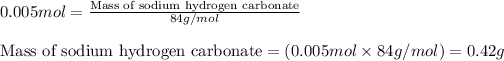
Hence, the mass of sodium hydrogen carbonate needed to completely neutralize stomach acid is 0.42 grams