Answer:
The correct answers are option 2, 3 and 6.
Step-by-step explanation:
Density is defined as mas of substance present in an unit volume of the substance.
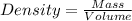
Density of same substance with different masses and volume remains the same that is it is an intensive property.
2. 20.2 g silver placed in 21.6 mL of water and 12.0 g silver placed in 21.6 mL of water.
3. 15.2 g copper placed in 21.6 mL of water and 50.0 g copper placed in 23.4 mL of water.
6. 11.2 g gold placed in 21.6 mL of water and 14.9 g gold placed in 23.4 mL of water.
Since the metal kept in both the cases are same.And metal has fix value of density , so from the given options the the option with sets of same of metals has equal densities.