Answer:
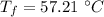
Step-by-step explanation:
Given:
- mass of aluminium,
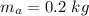
- initial temperature of the aluminium cylinder,
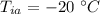
- mass of coffee,
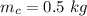
- initial temperature of coffee,
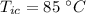
- specific heat of coffee (assuming water),
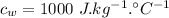
- specific heat of aluminium,
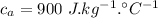
When the coffee and the aluminium cylinder come in contact then heat released by the coffee is equal to the heat gained by the aluminium.
Mathematically:
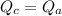
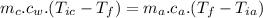
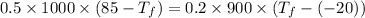
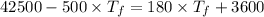
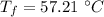 is the final temperature of agreement.
is the final temperature of agreement.