Answer:
1.00 mole of He have
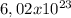 atoms.
atoms.
Step-by-step explanation:
To solve this exercise it is important to know the definition of Avogadro's number
Avogadro's number is the proportional factor that relates the molar mass of a substance to the number of elementary units (atoms, molecules, particles, ions, electrons) that constitute it and its magnitude is equal to 6.022 140 857 × 10²³
One mole of helium we have
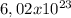 atoms.
atoms.