Answer:
The increasing order of masses of molecule ions:
118 g/mol(75.8%) , 120 g/mol(24.2%)
Step-by-step explanation:
Chlorine occurs as 35-Cl (75.8%) and 37-Cl (24.2%).
Atomic mass of 35-Cl = 35 g/mol
Atomic mass of 37-Cl = 37 g/mol
Mass of Chlorocyclohexane in which 35-cl is present as a chlorine atom:
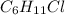
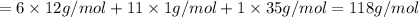
Mass of Chlorocyclohexane in which 37-Cl is present as a chlorine atom:
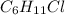
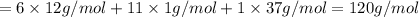
The increasing order of masses of molecule ions:
118 g/mol(75.8%) < 120 g/mol(24.2%)