Answer:
4.63 g/mL is the density of uranium hexafluoride.
Step-by-step explanation:
Mass of an uranium hexafluoride , m=
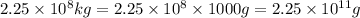
1 kg = 1000 g
Volume of drum in which uranium hexafluoride was stored =
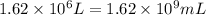
1 L = 1000 mL
Volume of uranium hexafluoride stored in 30 drums = V
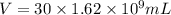
Density of an uranium hexafluoride =d
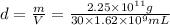
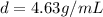
4.63 g/mL is the density of uranium hexafluoride.