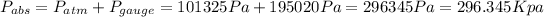Answer:
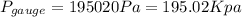
And the absolute pressure at the bottom would be:
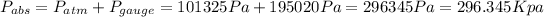
Explanation:
From the figure attached we know the following two conditions:
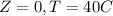
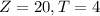
And we want a linear model like this one:
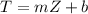
Where m abd b represent the slope and intercept.
We can find the slope with the following expression:
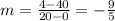
And then we can find the intercept using the first point:
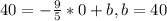
So then the linear model is:
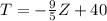
Assuming the following formula for the density:
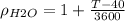
If we replace the expression for T into the density we got:
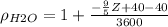
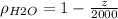
Since the units for
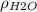 are g/ml we can convert this into kg/m^3 multiplying by 1000 and we got:
are g/ml we can convert this into kg/m^3 multiplying by 1000 and we got:
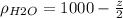
Then we have the following differential equation:
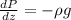
If we replace the expression for the density in terms of z we got:
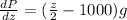
And we can separate like this:

Now w can integrate both sides and we got:
![\Delta P = g[((\Delta z)^2)/(4) -1000\Delta z]](https://img.qammunity.org/2021/formulas/mathematics/college/9vm8c4rkc251ftl5dfxt068uidv0dp4wjl.png)
And that would be the expression for the change of pressure:
For our case we have 20m of difference between the surface and the bottom so then the change of pressure between these two levels is:
![\Delta P= 9.8 (m)/(s^2) [((20m)^2)/(4) -1000(20m)]= -195020 Pa](https://img.qammunity.org/2021/formulas/mathematics/college/lkesj1lt577vg46wji2u4gedcltkkjuvhv.png)
We know that the pressure increase with the depth. So then the gage pressure at the bottom would be:
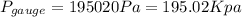
And the absolute pressure at the bottom would be: