Answer:
Basic
Explanation:
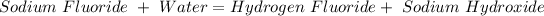
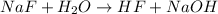
Sodium fluoride, NaF, is a soluble salt that dissociates completely in aqueous solution to give sodium cations, Na+, and fluoride anions, F-
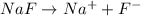
and when it dissolve in water the pH of the solution becomes greater than seven thereby becoming basic.