The new pressure is 2 Kpa
Solution:
At constant temperature, the pressure of a sample of gas is inversely proportional to its volume
Therefore,
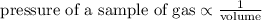
Let "p" be the pressure of sample gas
Let "v" be the volume
Then we get,

Where, "k" is the constant of proportionality
I have some oxygen in a 2.28 liter container with a pressure of 5 kPa
Substitute v = 2.28 and p = 5 in eqn 1
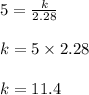
I move all of it to a 5.7 liter container at the same temperature
Substitute k = 11.4 and v = 5.7 in eqn 1
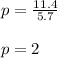
Thus new pressure is 2 kPa