Answer: The concentration of calcium, magnesium, sodium and potassium are
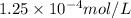 ,
,
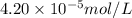 ,
,
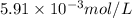 and
and
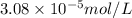 respectively
respectively
Step-by-step explanation:
To convert the mass from milligrams to grams, we use the conversion factor:
1 g = 1000 mg
To convert the given concentration fro grams to moles, we use the equation:
 .....(1)
.....(1)
Concentration given = 5.0 mg/L =
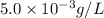
We know that:
Molar mass of calcium element = 40 g/mol
Putting values in equation 1, we get:
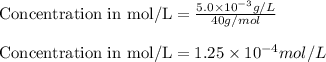
Concentration given = 1.0 mg/L =
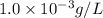
We know that:
Molar mass of magnesium element = 24 g/mol
Putting values in equation 1, we get:
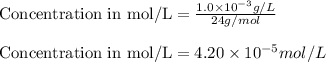
Concentration given = 136 mg/L =
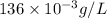
We know that:
Molar mass of sodium element = 23 g/mol
Putting values in equation 1, we get:
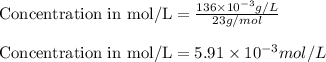
Concentration given = 1.2 mg/L =
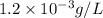
We know that:
Molar mass of potassium element = 39 g/mol
Putting values in equation 1, we get:
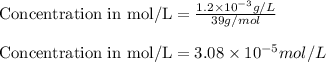
Hence, the concentration of calcium, magnesium, sodium and potassium are
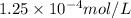 ,
,
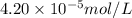 ,
,
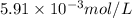 and
and
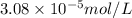 respectively
respectively