Answer:
10g Carbon has more particles.
Step-by-step explanation:
To Find
Which has more particles 10g of C or 10g of Ca
The molar mass of carbon is 12g and Calcium is 40g.
1 mole of atom , molecule or ions contain Avagadro number (6.022×10∧23) of particles and also have a mass equal to it's molar mass.
For C,
12g C will have 6.022×10∧23 atoms
10g C will have
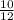 ×6.022×10∧23 atoms
×6.022×10∧23 atoms
=0.833 × 10∧23 atoms
For Ca,
1 mole Ca =40g
40g Ca will have 6.022×10∧23 atoms
10g Ca will have
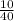 ×6.022×10∧23 atoms
×6.022×10∧23 atoms
=0.25 × 6.022×10∧23 atoms