Answer:
Total 20 mL of solution is needed in which 2.5 m L will be of Albuterol solution and 17.5 mL will be of normal saline solution.
Step-by-step explanation:
Amount of Albuterol in 1 vial = 2.5 mg/0.5 mL
Volume of dose in which 12.5 mg of Albuterol is present be x.
So,
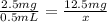
x = 2.5 mL
Volume of Albuterol solution is 2.5 mL.
If the output flow of your continuous nebulizer is 10 mL per hour.Then in 2 hours total volume of solution delivered = T
T = 10 × 2 mL = 20 mL
Volume of normal saline solution needed = y
T = x + y
y = T - x = 20 mL - 2.5 mL = 17.5 mL
Total 20 mL of solution is needed in which 2.5 mL will be of Albuterol solution and 17.5 mL will be of normal saline solution.