Answer:
The electron dot structure of
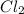
Step-by-step explanation:
Step 1: First we have to know the number of valence electrons present in a molecule.
Chlorine is an element from group 17 with 7 nos. of valence electrons.
So, the given
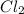 will have 7+7 = 14
will have 7+7 = 14
Step 2: We, will begin by drawing the skeleton of the molecule.
Step 3: Then, we have to place the bonding pairs of the electrons in the middle atoms so that chemical bonds can be formed.
Step 4: The electrons left will be used as lone pairs for central and terminal atoms in order to achieve an octet and to finish the electron dot structure. The structural formula and electron dot structure has been attached below.