The question is incomplete, here is the complete question:
What is the mass % of ammonium chloride in a 1.73 M ammonium chloride aqueous solution at 20 °C? The density of the solution is 1.0257 g/mL
Answer: The mass percent of ammonium chloride in solution is 9.03 %
Step-by-step explanation:
We are given:
Molarity of ammonium chloride solution = 1.73 M
This means that 1.73 moles of ammonium chloride is present in 1 L or 1000 mL of solution.
- To calculate the mass of solution, we use the equation:
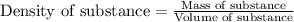
Density of solution = 1.0257 g/mL
Volume of solution = 1000 mL
Putting values in above equation, we get:
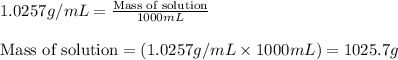
- To calculate the number of moles, we use the equation:

Moles of ammonium chloride = 1.73 moles
Molar mass of ammonium chloride = 53.5 g/mol
Putting values in above equation, we get:
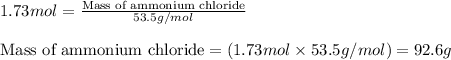
- To calculate the mass percentage of ammonium chloride in solution, we use the equation:
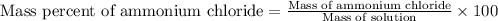
Mass of solution = 1025.7 g
Mass of ammonium chloride = 92.6 g
Putting values in above equation, we get:
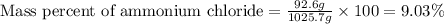
Hence, the mass percent of ammonium chloride in solution is 9.03 %