Answer:
The solubility of the organic amide product in the solvent hexane is 0.0403 moles/L at 0° C
Step-by-step explanation:
When 2.85gm dissolved in 20ml of boiling solvent
After re crystallization 2.62gm was obtained
so, that the dissolved amount at 0° C is
2.85 - 2.62
= 0.23 gm in 20ml hexane
Mole of amide
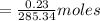
Solubility
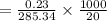
= 0.0403 moles/L
Therefore, the solubility of the organic amide product in the solvent hexane is 0.0403 moles/L at 0° C