Answer:
Yes, the unknown substance is the liquid ethylene glycol.
Step-by-step explanation:
Here the density of the unknown substance is 69.22lb/f and the density of ethylene glycol, the liquid used in antifreeze is 1.1088g/ml
We know, 1g = 0.0022lb
1ml=
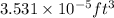
Therefore, 1g/ml = 62.428
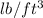
Since, the density of ethylene glycol = 1.1088g/ml
= 1.1088
 62.428
62.428
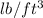
= 69.22
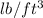
When the density of ethylene gas is converted from g/ml unit to
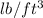 unit, it is found to have the same value (69.22)Therefore, the unknown substance here is liquid ethylene glycol.
unit, it is found to have the same value (69.22)Therefore, the unknown substance here is liquid ethylene glycol.