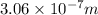This is an incomplete question, the image for the given question is attached below.
Answer : The wavelength of photon would be absorbed,
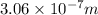
Explanation :
From the given diagram of energy we conclude that,
Energy at ground state, A = 400 zJ
Energy of 2nd excited state, C = 1050 zJ
Now we have to calculate the energy of the photon.
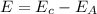
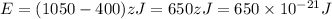
Now we have to calculate the wavelength of the photon.
Formula used :
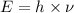
As,
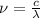
So,
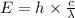
where,
E = energy of photon =
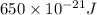
 = frequency of photon
= frequency of photon
h = Planck's constant =
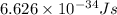
 = wavelength of photon = ?
= wavelength of photon = ?
c = speed of light =
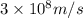
Now put all the given values in the above formula, we get:
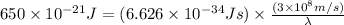
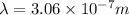
Therefore, the wavelength of photon would be absorbed,