Answer:
C Fe³⁺ = 0.00109 M
Step-by-step explanation:
The reaction between Fe(NO₃)₃ and KSCN is:
Fe³⁺(aq) + SCN⁻(aq) → FeSCN²⁺(aq)
5.9mL 4.4mL
0.0019M 0.0045M
The moles (η) of Fe(NO₃)₃ are:
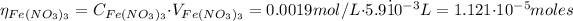
Since in 1 mol of Fe(NO₃)₃ we have 1 mol of Fe³⁺, the moles of Fe³⁺ are:
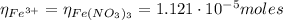
Now, to calculate the initial concentration of Fe³⁺ we need first to find the total volume in the final solution:
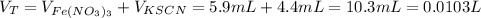
Finally, the the initial concentration of Fe³⁺ is:
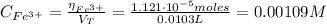
I hope it helps you!