Answer:
The answer to your question is 32.44 moles
Step-by-step explanation:
Data
moles of Na₂CO₃ = ?
volume = 9.54 l
concentration = 3.4 M
Formula
Molarity =
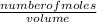
Solve for number of moles
number of moles = Molarity x volume
Substitution
Number of moles = (3.4)( 9.54)
Simplification
Number of moles = 32.44