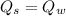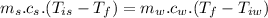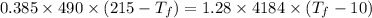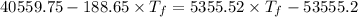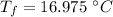Answer:
Step-by-step explanation:
Given:
- mass of water,
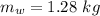
- initial temperature of water,
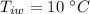
- mass of steel,
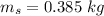
- initial temperature of steel,
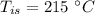
- specific heat capacity of steel,
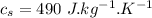
- specific heat capacity of water,
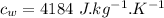
Now by the law of conservation of energy, the heat released by the steel is equal to the heat absorbed by the water: