Answer:
The volume of the solution is 190 milliliters.
Step-by-step explanation:
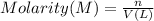
Where :
n = moles of compound
V = Volume of solution in L
M = Molarity of solution
Moles of nickel(II) chloride = n = 175 milli mol = 0.175 mol
1 milli mol = 0.001 mol
Volume of nickel(II) chloride solution = V
Molarity of the nickel(II) chloride = 0.92 M
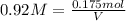
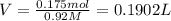
1 L = 1000 mL
V = 0.1902 L= 0.1902 × 1000 mL = 190.2 mL ≈ 190 mL
The volume of the solution is 190 milliliters.