Answer: The percentage abundance of
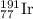 and
and
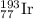 isotopes are 37.10% and 62.90% respectively.
isotopes are 37.10% and 62.90% respectively.
Step-by-step explanation:
Average atomic mass of an element is defined as the sum of masses of each isotope each multiplied by their natural fractional abundance.
Formula used to calculate average atomic mass follows:
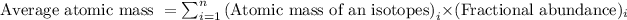 .....(1)
.....(1)
Let the fractional abundance of
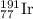 isotope be 'x'. So, fractional abundance of
isotope be 'x'. So, fractional abundance of
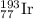 isotope will be '1 - x'
isotope will be '1 - x'
- For
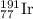 isotope:
isotope:
Mass of
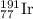 isotope = 190.9606 amu
isotope = 190.9606 amu
Fractional abundance of
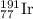 isotope = x
isotope = x
- For
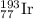 isotope:
isotope:
Mass of
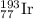 isotope = 192.9629 amu
isotope = 192.9629 amu
Fractional abundance of
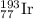 isotope = 1 - x
isotope = 1 - x
Average atomic mass of iridium = 192.22 amu
Putting values in equation 1, we get:
![192.22=[(190.9606* x)+(192.9629* (1-x))]\\\\x=0.3710](https://img.qammunity.org/2021/formulas/chemistry/college/8e9r08t2nvxorzdi6d4oym4j3gzgaplo63.png)
Percentage abundance of
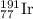 isotope =
isotope =
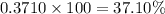
Percentage abundance of
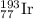 isotope =
isotope =
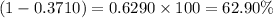
Hence, the percentage abundance of
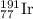 and
and
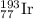 isotopes are 37.10% and 62.90% respectively.
isotopes are 37.10% and 62.90% respectively.