Compounds have the lowest percentage of gold content by weight is Aui3
M(Au) =197g/mol
M(O) =16g/mol
M(H)=1g/mol
M(Cl)=35g/mol
M(I)=53 g/mol
1) M=214g/mol =197+1+16
197/214= 0.921
2) M=248g/mol =
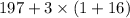
197/248=0.794
3) M=302g/mol =
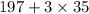
197/302=0.652
4) M=356g/mol =
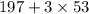
197/356=0.5533
Step-by-step explanation:
Iodine is the heaviest out of I, Cl and OH. The items on your list hold less and less gold by mass as you go down. The gold dissolution percentage in a hypochlorite-iodide mix-up is completely conditioned upon the solution pH. So the iodine has the lowest gold content percentage.