Answer:
A solution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 mL solution. The weight/weight % or percent by mass of the solute is :
2.67%
Step-by-step explanation:
Note : Look at the density of potassium nitrate in water if given in the question.
You are calculating weight /Volume not weight/weight % or percent by mass of the solute
Here the weight/weight % or percent by mass of the solute is asked : So first convert the VOLUME OF SOLUTION into MASS
Density of potassium nitrate in water KNO3 = 2.11 g/mL
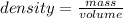
Density = 2.11 g/mL
Volume of solution = 86.4 mL
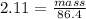
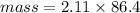
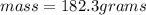
Mass of Solute = 4.87 g
Mass of Solution = 183.2 g
w/w% of the solute =
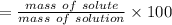
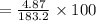
w/w%=2.67%