Answer: The volume of acid and water that must be mixed will be 4.8 L and 11.2 L
Step-by-step explanation:
We are given:
Volume of mixture = 16 L
Percent of acid present = 30 %
Calculating the percentage of acid present in the mixture:
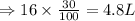
The mixture is made entirely of acid and water.
Volume of acid in the mixture = 4.8 L
Volume of water in the mixture = 16 - 4.8 = 11.2 L
Hence, the volume of acid and water that must be mixed will be 4.8 L and 11.2 L