Answer:
initial and final temperatures of both the water and metal, mass of the metal, and mass of the water
Step-by-step explanation:
Heat lost by the metal,
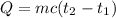
Heat gained by the water in the calorimeter,
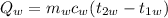
For energy to be conserved in the system, the heat lost by the metal will equal the heat gain by the water in the calorimeter.
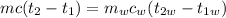
Where,
m is the mass of the metal
c is specific heat capacity of the metal
t₂ is the final temperature of the metal
t₁ is the initial temperature of the metal
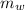 is the mass of the water
is the mass of the water
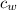 is specific heat capacity of water
is specific heat capacity of water
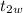 is the final temperature of water
is the final temperature of water
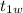 is the initial temperature of water
is the initial temperature of water
From the question given, specific heat capacity of the water is known, the quantities to be measured are;
Initial and final temperatures of both the water and metal,
Mass of the metal, and mass of the water