Answer:
The answer to your question is 280 g of Mg(NO₃)₂
Step-by-step explanation:
Data
Efficiency = 30.80 %
Mg(NO₃)₂ = ?
Magnesium = 147.4 g
Copper (II) nitrate = excess
Balanced Reaction
Mg + Cu(NO₃)₂ ⇒ Mg(NO₃)₂ + Cu
Reactants Elements Products
1 Mg 1
1 Cu 1
2 N 2
6 O 6
Process
1.- Calculate the theoretical yield
Molecular weight Mg = 24
Molecular weight Mg(NO₃)₂ = 24 + (14 x 2) + (16 x 6)
= 24 + 28 + 96
= 148 g
24 g of Mg -------------------- 148 g of Mg(NO₃)₂
147.4 g of Mg ------------------- x
x = (147.4 x 148) / 24
x = 908.96 g of Mg(NO₃)₂
2.- Calculate the Actual yield
yield percent =
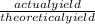
Solve for actual yield
Actual yield = Yield percent x Theoretical yield
Substitution
Actual yield =
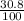 x 908.96
x 908.96
Actual yield = 279.95 ≈ 280g