Answer:
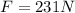
Step-by-step explanation:
Let's use the electrostatic force equation to find it.
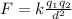
Here:
- q₁ and q₂ are the same, both are the proton charges (
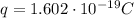 ).
). - r is the distance between two protons. In this case, it will the diameter of the He nucleus (d=10⁻¹⁵m).
- k is the electrostatic constant (
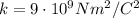 )
)
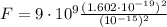
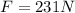
I hope it helps you!