This is an incomplete question, here is a complete question.
Consider the reaction A+B ⇌ C+D. We will assume that both the forward and reverse reactions are elementary processes and that the value of the equilibrium constant is very large.
Which species predominate at equilibrium, reactants or products?
Answer : The species predominate at equilibrium is, products.
Step-by-step explanation:
Equilibrium reaction : It is a reaction which do not go on completion that means the reactant forms product and the products goes back to the reactants simultaneously.
Equilibrium constant : It is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
In a chemical equilibrium reaction, the equilibrium state is achieved when the rate of forward reaction becomes equals to the rate of the backward reaction.
There are 3 conditions:
When
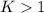 ; the reaction is product favored.
; the reaction is product favored.
When
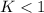 ; the reaction is reactant favored.
; the reaction is reactant favored.
When
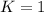 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
As, the value of equilibrium constant is very large that means K > 1. So, the reaction is product favored that means products are predominant.
Hence, the species predominate at equilibrium is, products.