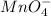This is an complete question, here is a complete question.
Consider the following standard reduction potentials in acid solution:
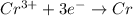

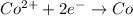

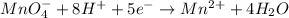
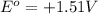
The weakest reducing agent listed above is:
(a)
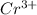
(b)
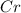
(c)
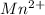
(d)
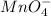
Answer : The correct option is, (d)
Explanation :
As we know that,
The substance having highest positive
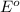 potential will always get reduced and will undergo reduction reaction and weakest reducing agent or strongest oxidizing agent.
potential will always get reduced and will undergo reduction reaction and weakest reducing agent or strongest oxidizing agent.
From the standard reduction potentials we conclude that,
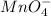 having highest positive
having highest positive
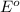 potential than the other. So, it will always get reduced and will undergo reduction reaction and weakest reducing agent.
potential than the other. So, it will always get reduced and will undergo reduction reaction and weakest reducing agent.
Hence, the correct option is, (d)