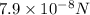This is an incomplete question, here is a complete question.
In the Bohr model of the hydrogen atom, an electron moves in a circular path around a proton. The speed of the electron is approximately
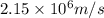 ?
?
Find the force acting on the electron as it revolves in a circular orbit of radius
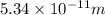 .
.
Answer : The force acting on electron is,
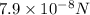
Explanation :
Formula used :
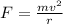
where,
F = force acting on electrons
m = mass of electrons =
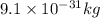
v = speed of electron =
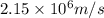
r = radius of circular orbit =
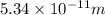
Now put all the given values in the above formula, we get:
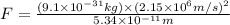
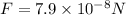
Thus, the force acting on electron is,