Answer:
b. 18.6
Step-by-step explanation:
Given that:-
Mass of silicon dioxide = 35.0 g
Molar mass of silicon dioxide = 60.08 g/mol
The formula for the calculation of moles is shown below:
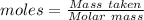
Thus,
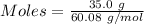
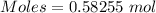
From the formula,
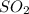 1 mole of sulfur dioxide contains 2 moles of oxygen
1 mole of sulfur dioxide contains 2 moles of oxygen
So,
0.58255 mole of sulfur dioxide contains 2*0.58255 moles of oxygen
moles of oxygen = 1.1651 moles
Molar mass of oxygen = 15.999 g/mol
Mass= Moles*Molar mass =
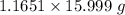 = 18.6 g
= 18.6 g