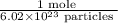 or
or
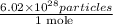 is the conversion factor used to convert mole to particle.
is the conversion factor used to convert mole to particle.
Step-by-step explanation:
The Avogadro's number named after the scientist "Amedeo Avogadrothe" and represented as N or N 0. It is the number of constituent particles like atoms, molecules or ions which are present in one mole of salt or compound.
It is the dimensionless and international unit of amount of substance given as
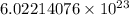 . The number of particles like atoms, molecules or formula units can be converted to moles by dividing the given particle value by Avogadro's number. . For example, if u want to convert
. The number of particles like atoms, molecules or formula units can be converted to moles by dividing the given particle value by Avogadro's number. . For example, if u want to convert
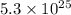 molecules of
molecules of
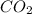 into moles, then have to do as below,
into moles, then have to do as below,
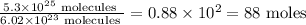
The value of the Avogadro constant has been selected for all experimental purpose so that the mass of 1 mole of a chemical compound (in grams) is numerically equal to the average mass of one molecule of the compound.