Answer:
1) 11.64 mol/L is the molarity of concentrated HCl.
2) 135.40 mL of volume of 11.64 M will need to prepare 985 mL of 1.6 M HCl.
3) 1,711.08 grams of sodium bicarbonate would be needed to neutralize the spill HCl solution.
Step-by-step explanation:
HCl solution with 36.0% HCl by mass, menas that in 100 g of solution 36.0 gram of HCl is present.
Mass of HCl= 36.0 g
Moles of HCl =
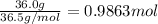
Mass of solution ,m= 100 g
Volume of solution = V = ?
Density of the solution ,d= 1.18 g/mL
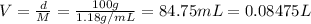
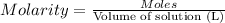
Molarity of the solution :
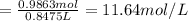
11.64 mol/L is the molarity of concentrated HCl.
2)
 ( Dilution equation)
( Dilution equation)
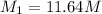
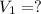
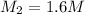
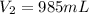
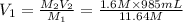
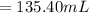
135.40 mL of volume of 11.64 M will need to prepare 985 mL of 1.6 M HCl.
3.
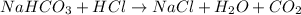
Concentration of HCl solution = 11.64 M
Volume of the HCl solution = 1.75 L
Moles of HCl in 1.75 L solution = n
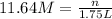
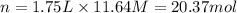
According to reaction 1 mole of HCl neutralized by 1 mole of sodium carbonate.
Then 20.37 moles of HCl will neutralized by ;
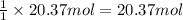 of sodium carbonate
of sodium carbonate
Mass of 20.37 moles of sodium carbonate :
= 20.37 mol × 84g/mol = 1,711.08 g
1,711.08 grams of sodium bicarbonate would be needed to neutralize the spill HCl solution.