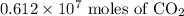 are present in
are present in
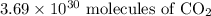
Step-by-step explanation:
It is known that each mole of an element is composed of avagadro's number of molecules. So if we need to determine, we need to divide the number of molecules with the avagadro's number.
So,
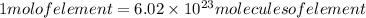
As here
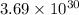 molecules of carbon di oxide is given. So the moles in it will be
molecules of carbon di oxide is given. So the moles in it will be
No. of moles of carbon dioxide =
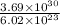
No. of moles =
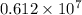 moles of carbon dioxide.
moles of carbon dioxide.
Thus,
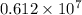 of carbon dioxide are present in
of carbon dioxide are present in
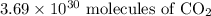 .
.