No, Silver will not react with dilute sulfuric acid.
Step-by-step explanation:
As Silver has the least reactivity, it is not capable to reduce hydrogen ion from sulfuric acid, even when the acid is in concentrated state. But if the concentrated acids are heated then the Silver may form Ag+ ions.
But silver (Ag) can react with the hot concentrated sulfuric acids (
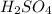 ). As mentioned in below to equation.
). As mentioned in below to equation.
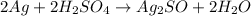
So if dilute sulfuric acid is used then there will not be any kind of reaction with silver ions.