Answer:
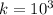
Step-by-step explanation:
k stand for equilibrium constants in terms of reaction
The higher the value of an equilibrium constant the faster the equilibrium reaction comes to completion.
Consider the example below:
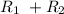 ⇄
⇄
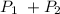
where
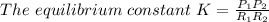
For a faster reaction the numerator i.e. the right hand side of the equation have to be higher than the left hand side (the denominator). therefore the higher the numerator, the higher the value of the equilibrium constant and the faster the reaction get to completion thus option c is correct.