Answer:
d) tetrahedral
Step-by-step explanation:
Given that the central atom is attached to the four group.
Number of bond pairs = 4
Number of lone pairs = 0
The formula is
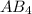 .
.
According to VSEPR theory,
The central atom has 4 bond pairs and 0 lone pair. Hybridization is sp³. The geometry is tetrahedral which has an angle of 109.5°.
Methane,
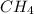 posses a tetrahedral geometry as four atoms of hydrogen are attached to the central atom which is carbon.
posses a tetrahedral geometry as four atoms of hydrogen are attached to the central atom which is carbon.
Hence. D option is correct.