Answer:
Kc = 1.27x10⁻⁴ mol²/L²
1.27x10⁻⁴ * 10⁶ = 127
Step-by-step explanation:
When a reaction achieves the equilibrium, the velocity of the formation of the products is equal to the velocity of the formation of the reactants, thus, the concentration and the partial pressure of the components are constant in equilibrium.
To characterize it, we may calculate a constant, which depends on the activity of the components. The activity can be a substitute for the concentration, thus the constant is called Kc, or by the pressure, and the constant is called Kp. These constant only varies with the temperature.
Because the activity of solids is equal to 1, the solids components are not placed in the constant equilibrium equation. And for Kp, only gaseous components are taken into consideration. For a general reaction:
aA + bB ⇄ cC + dD
![Kc = ([C]^c*[D]^d)/([A]^a*[B]^b)](https://img.qammunity.org/2021/formulas/chemistry/college/8xa4xoted8xo43kjtma19am5vwwwwb7zis.png)
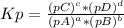
Where [X] is the concentration of X, and pX is the partial pressure of X.
These constant are related by the Clayperion equation:
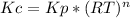
Where R is the gas constant (0.082 atm.L/mol.K), T is the temperature (210°C = 483 K), and n is the variation of the gases reaction coefficientes (n = (a+b) - (c-d)), in this gase only the products are gases, so n = -2.
Kc = 0.2*(0.082*483)⁻²
Kc = 0.2*(39.606)⁻²
Kc = 1.27x10⁻⁴ mol²/L²
1.27x10⁻⁴ * 10⁶ = 127