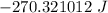Answer:
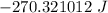
Step-by-step explanation:
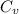 = Heat capacity of Hg = 28 J/mol
= Heat capacity of Hg = 28 J/mol
 = Change in temperature =
= Change in temperature =
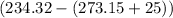
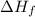 = Enthalpy of fusion = 2.29 kJ/mol
= Enthalpy of fusion = 2.29 kJ/mol
The number of moles is given by
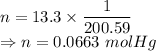
Heat is given by
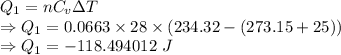
Heat released is given by
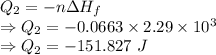
Total heat is given by
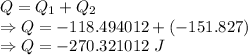
The total heat released is