The question is incomplete, here is the complete question:
During an exothermic chemical reaction, four moles of gaseous reactants are turned into two moles of gaseous products. Is this change spontaneous?
A. Yes
B. No
C. Can't decide with information given
Answer: The spontaneity of the reaction cannot be determined from the given data.
Step-by-step explanation:
For the reaction to be spontaneous, the Gibbs free energy of the reaction must come out to be negative.
The equation used to calculate Gibbs free energy follows:
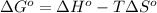
Exothermic reactions are defined as the reactions in which energy is released in the form of heat. The enthalpy change
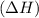 of the reaction comes out to be negative for this kind of reaction.
of the reaction comes out to be negative for this kind of reaction.
Entropy change is defined as the change in the measure of randomness in the reaction. It is represented as
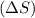 . Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
. Randomness of gaseous particles is more than that of liquid which is further more than that of solids.
As, number of gaseous particles on the product side is less than the number of gaseous particles on the reactant side. So, the entropy change is negative.
![\Delta G=-ve-[T(-ve)]\\\Delta G=-ve+T](https://img.qammunity.org/2021/formulas/chemistry/college/jowfke8wbxf1hu3xozkke5d06yhpjadonb.png)
As, the value of Gibbs free energy is dependent on temperature of the reaction. If the temperature is high, the reaction will be non-spontaneous and if the temperature is low, the reaction will be spontaneous.
Hence, the spontaneity of the reaction cannot be determined from the given data.