Answer:
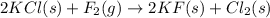
The formula of the products are :
KF = Potassium fluoride
Cl2 = Chlorine
Step-by-step explanation:
Single replacement reaction :
It is the reaction in which one element(More reactive) replace other element (less reactive) in the compound . These are represented by :
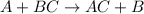
Here , the more Reactive A replaces the less reactive B from its compound BC.
Note , that A and B should be almost similar in properties.
Now , Chlorine and Fluorine are both Non - metals whereas Potassium is metal . So Potassium is not replaced in the reaction , since it is metal and only metal can replace other metals.
Chlorine is replaced by Fluorine in this reaction because The reactivity of F is greater than Cl.
The balanced equation is :
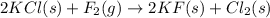
The reactants are :
KCl = Potassium Chloride
F2 = Fluorine
The formula of the products are :
KF = Potassium fluoride
Cl2 = Chlorine gas