To solve this problem we will apply the concept related to the heat transferred to a body to reach a certain temperature. This concept is shaped by the energy ratio of a body which is the product of the mass, its specific heat and the change in temperature. For the specific case, it will be the sum of the heat transferred to the Water, the Aluminum and the loss due to latency due to vaporization in the water. That is to say,
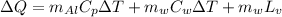
Here,
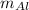 = Mass of Aluminum
= Mass of Aluminum
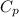 = Specific Heat of Aluminum
= Specific Heat of Aluminum
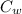 = Specific Heat of Water
= Specific Heat of Water
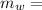 Mass of water
Mass of water
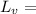 Latent of Vaporization
Latent of Vaporization
Replacing,
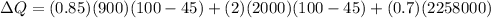
Converting,
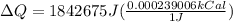
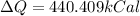
Therefore is required 440.409kCal