Answer:
η =0.856
Step-by-step explanation:
Given that
T₁ = 288 K
T₂ = 2000 K
We know that ,Carnot cycle is having 4 process ,in two are constant temperature process and other two are constant entropy process.
Heat rejection
Qr=T₁ ΔS
Heat addition
Qa=T₂ ΔS
We know that efficiency given as
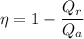
Now by putting the values
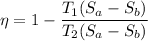
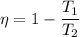
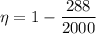
η =0.856