Answer : The average molecular mass of water vapor is, 18 g/mol
Explanation :
Molar mass : It is defined as the sum of the mass of all the atoms each multiplied its atomic masses that are present in the molecular formula of a compound. It is expressed in g/mol.
As we know that,
Atomic weight of hydrogen = 1 g/mole
Atomic weight of oxygen = 16 g/mole
Now we have to calculate the average molecular mass of
 .
.
Average molecular mass of
 =
=
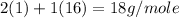
Therefore, the average molecular mass of water vapor is, 18 g/mol