Answer:
8 moles of water
Step-by-step explanation:
This is the combustion reaction and it is done by burning the substance in the presence of oxygen :
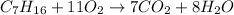
How to start :
First , note that every combustion reaction produce CO2 and H2O as products
So , unbalanced equation is :
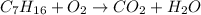
1. Balance the Carbon "C"
There are 7 carbon in C7H16 , so it will form same number of CO2.Hence , the reaction now become
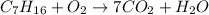
2. Balance Hydrogen"H"
There are 16 hydrogen in C7H16 ,
water(H2O) has 2 hydrogen , hence C7H16 produce 8 H2O
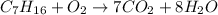
3. Balance the Oxygen only after balancing C and H
Count the number of Oxygen(O) in the products: 7CO2 + 8H2O
7 CO2 = 2(7) oxygen = 14 O
8H2O = 8(1) = 8 O
Total oxygen in product = 8 + 14 = 22
Total oxygen in reactant(O2) = 2
So we have to make 2 into 22
It is done by multiplying O2 with 11
Hence the balanced equation is :
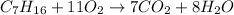
Check the elements are balanced or not on both side :
O= 22
H = 16
C= 7