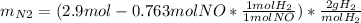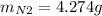Answer:
1) Maximun ammount of nitrogen gas:
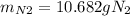
2) Limiting reagent:
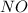
3) Ammount of excess reagent:
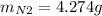
Step-by-step explanation:
The reaction

Moles of nitrogen monoxide
Molecular weight:
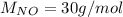
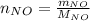
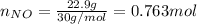
Moles of hydrogen
Molecular weight:
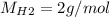
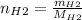
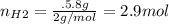
Mol rate of H2 and NO is 1:1 => hydrogen gas is in excess
1) Maximun ammount of nitrogen gas => when all NO reacted
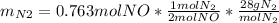
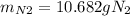
2) Limiting reagent:
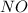
3) Ammount of excess reagent: